Introduction: Minimal residual disease (MRD) negativity is increasingly recognised as the optimal measure of therapeutic response for multiple myeloma (MM) patients and is associated with improved survival. Bone marrow (BM) assessment of MRD with next-generation sequencing (NGS) or next-generation flow cytometry (NGF) can achieve a minimum sensitivity of 10 -5 however an invasive procedure is required. Additionally, this single-site BM biopsy may be limited by specimen quality and failure to capture the spatial heterogeneity of MM. Peripheral blood-based mass spectrometry (PB-based MS) offers the potential for highly sensitive, non-invasive sequential MRD assessment. EasyM is a PB clonotypic MS assay involving de novo amino acid sequencing of the full-length M-protein and quantification of unique peptides with parallel reaction monitoring. EasyM has been compared to 8-colour multiparameter flow cytometry with a sensitivity of 10 -4 (Liyasova et al, Clin Cancer Res, 2021) and clonoSEQ (Slade et al, Blood, 2022), but not to NGF to our knowledge. In this context, we have evaluated EasyM in MM patients undergoing sequential NGF (EuroFlow platform) MRD assessment.
Methods: We retrospectively identified MM patients enrolled in the Australasian Leukaemia and Lymphoma Group MM19 (ACTRN12616000772448) and MM21 (ACTRN12618001490268) trials with measurable M-protein ≥2g/L by serum protein electrophoresis and/or free light chains ≥100mg/L at baseline. Briefly, the MM19 trial evaluated the addition of ixazomib to thalidomide and dexamethasone consolidation therapy for 12 months in transplant eligible newly diagnosed MM (TE NDMM) patients undergoing front-line autologous stem cell transplantation (ASCT), whilst the MM21 trial evaluated an intensive salvage approach using daratumumab-lenalidomide-dexamethasone (DRd) as re-induction (DRd x 4 cycles) and post-ASCT consolidation (DRd x 12 cycles followed by R maintenance until disease progression) in TE NDMM patients failing (<partial response as best response) front-line bortezomib-based induction therapy. NGF MRD status was determined using the standardised 8-colour EuroFlow platform at pre-ASCT, post-ASCT, and end of consolidation timepoints in MM19 and MM21 patients and additionally post-cycle 2 of consolidation in MM21 patients. Matched serum samples were evaluated with the EasyM assay. Concordance between NGF and MS was assessed by the Chi-squared and McNemar's tests.
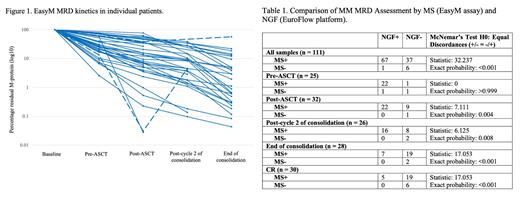
Results: A total of 43 patients were identified for analysis, with 9 MM19 and 34 MM 21 patients. 1 patient was not sequenced due to low baseline values and 2 patients had no clonotypic target tryptic peptides to monitor MRD. EasyM was undetectable (MS-) in 3 (8%) patients. 2 patients achieved MS- pre-ASCT and the third achieved MS- post-cycle 2 of consolidation. All 3 patients remain in complete response (CR) at 44-48 months post-ASCT. Sequential MS (Figure 1) revealed rising EasyM levels in 2 patients, preceding relapse by 15 and 25 months; of note, 1 patient had matched BM and was NGF MRD negative. Serum samples for MS with matched BM for NGF (Table 1) were available for 25 patients pre-ASCT, 32 post-ASCT, 26 post-cycle 2 of consolidation, and 28 end of consolidation with NGF MRD negativity (NGF-) of 8%, 31%, 38%, and 75% at these timepoints, respectively. Of the 111 matched samples in total, 43 (39%) were NGF- but only 7 (6%) were MS-, and 73 (66%) were concordant. The Chi-squared test for association between MS and NGF was not significant at all 4 timepoints. The McNemar's test p-values were >0.999, 0.004, 0.008, and <0.001 for the 4 timepoints, indicating that MS+/NGF- were more likely than MS-/NGF+ discordances at all timepoints except for pre-ASCT. Of the 30 matched samples with confirmed CR status, 5 (17%) were MS+/NGF+, 19 (63%) were MS+/NGF-, and 6 (20%) were MS-/NGF- (Chi-squared and McNemar's p-values of 0.549 and <0.001).
Conclusion: This preliminary data highlights the potential of EasyM for sequential PB-based clonotypic MS MRD monitoring in MM. Concordance with standard BM NGF was poor, with 63% of samples with confirmed CR showing detectable M-protein by EasyM but NGF MRD negativity, consistent with the higher sensitivity of EasyM. Comparison of larger sample sets and validation through prospective clinical trials is warranted to better assess the clinical utility of EasyM and rationalise BM-based assessment for MM patients.
Disclosures
Khaled:Rapid Novor Inc: Current Employment. McDonald:Rapid Novor Inc.: Current Employment. Reynolds:Abbvie: Current equity holder in publicly-traded company, Research Funding; HemaLogix: Consultancy; Novartis AG: Current equity holder in publicly-traded company; Novartis Australia: Honoraria; Alcon AG: Current equity holder in publicly-traded company. Motorna:Antengene: Consultancy. Quach:Karyopharm: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: receipt of study materials, Research Funding; GSK: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: receipt of study materials; Leadership or fiduciary role, Research Funding; Sanofi: Consultancy, Other: receipt of study materials; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Leadership or fiduciary role. Yang:Rapid Novor Inc.: Current Employment, Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees. Spencer:Antengene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Amgen: Consultancy, Honoraria; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Haemalogix: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Honoraria, Research Funding, Speakers Bureau; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; IDP Pharma: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal